Throughout the past week, I have been building on the research that I did last week on the disassembly step for waste printed circuit board (WPCB) recycling while expanding into the recovery step of recycling. While the usage of supercritical water for the degradation of organic materials in WPCBs was promising, the energy required to reach the supercritical temperature and pressure doesn’t appear to be viable on an industrial scale. In my research, however, I found an article (Degradation of brominated epoxy resin and metal recovery from waste printed circuit boards through batch sub/supercritical treatments, 2013) about a similar process with more promising conditions through the use of sub-supercritical water.
While a supercritical fluid can only be maintained by reaching the critical point for both temperature and pressure, sub-supercritical fluid can have similar properties to supercritical fluid while requiring only one of the conditions to be met. In the article, the researchers utilized sub-supercritical water heated to approximately 400 oC and significantly lower pressure than supercritical water to perform the degradation of organic compounds. The article described that, under those conditions, the researchers were able to obtain a debromination rate of approximately 97% from a 10g sample. This method of using sub-supercritical water to remove the organic compounds from WPCBs is promising, as it would remove the necessity of an extreme pressure, greatly reducing the energy cost of the process while eliminating the need of a chamber able to withstand extreme pressures. By removing the requirement of a high-pressure chamber, the degradation of organic compounds in WPCBs has increased viability to be moved from a lab environment to an industrial scale.
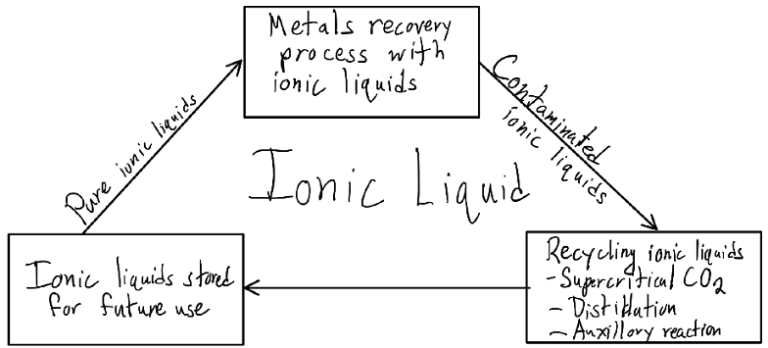
In addition to my research in the disassembly step of WPCB recycling, I also looked into the viability of ionic liquids in the recovery process. While ionic liquids don’t create emissions in recovering metals from WPCBs, the liquids themselves become contaminated with residue. When this contamination is combined with the high cost of producing task-specific ionic liquids, their viability to be done outside of a lab environment dwindles. One article I found however, described the ability for ionic liquids to be purified and recycled while still retaining reaction efficiency. While still expensive to make ionic liquids, the viability of recycling the used liquid reduces their cost over time compared to continuously buying other compounds as material for the reactions to recover metals done through hydrometallurgy. One such method discussed was through supercritical carbon dioxide due to its interesting properties to dissolve ionic liquids into its ions as a supercritical fluid but not as a gas, allowing for easy separation from contaminants.
Citation:
Xing, Mingfei, Zhang, Fu-Shen. “Degradation of brominated epoxy resin and metal recovery from waste printed circuit boards through batch sub/supercritical water treatments.” Chemical Engineering Journal, vol. 219, 2013, pp. 131-136.