Last week’s research into using subcritical water in the debromination process in separating the metals of printed circuit boards from the plastics. While this did mean that the same process that uses supercritical water could be done with less energy to maintain the pressure needed for a supercritical state, massive amounts of energy would still be used in heating the water to the required temperature. After finding an article on salt deposition in supercritical water, I realized that there might be a possibility to maintain the required temperature while reducing the energy needed to reach that point.
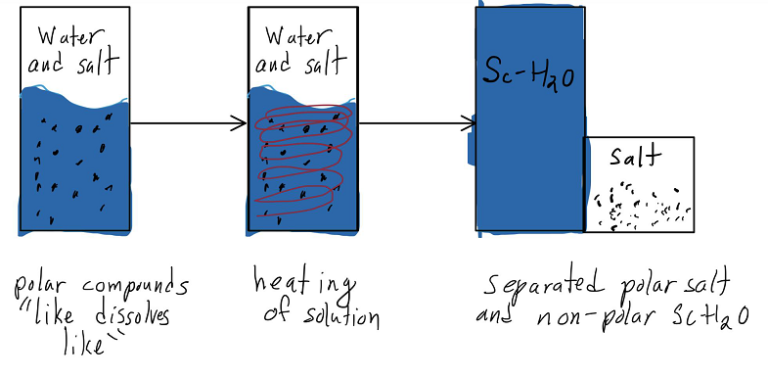
Salts in water demonstrate multiple beneficial properties that may assist in heating water to the needed point. The most important characteristics are that salts are polar compounds and lower the specific heat of water. Specific heat denotes the energy needed to raise the temperature of one kilogram of material by one degree celsius. Lowering the specific heat of water in torn lowers the energy needed to get the water to the needed temperature, increasing the energy efficiency of the process. This concept in itself could be done with other materials, but salts in particular are effective due to their polar properties. As a polar material, salts have a neutral overall charge, but also have regions within the compound with different charges. Similarly, water is also a polar compound, and, due to the chemical rule of “like dissolves like,” salts dissolve in water. The importance of salt polar characteristics comes into play when water reaches a subcritical state with enough temperature. As the temperature of water increases, its polarity decreases, essentially becoming a nonpolar compound with enough heat. As the salt and water molecules are now not alike, the salt molecules precipitate out of the water, leaving the salt that was put in and leaving the now heated water. Through this concept, it may be able to have a minimal material loss method of reducing the energy needed to heat water for industrial purposes. Unfortunately, it doesn’t seem like an experiment has been done in this concept yet, but further research will be done.